NEW! ALPS Hybrid 700 Series for BFS/FFS Technology 
Designed and built with 43 years of experience & customer-focused approach.
ALPS 600 & 300 Series 
.
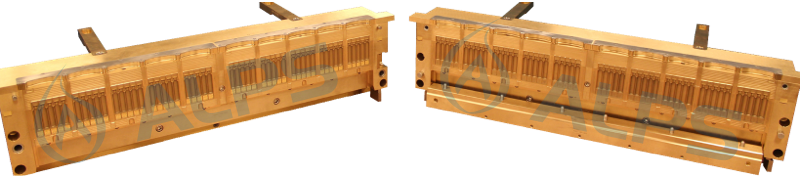
ALPS Molds 
Examples of ALPS Molds For 700 Series Machines.
Isolation Insertion Technology (IIT) 
ALPS Patented Deflasher

2mL - 5mL Vials
Up to 15,000/Hr
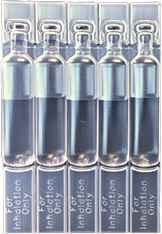
Respiratory/Inhalation
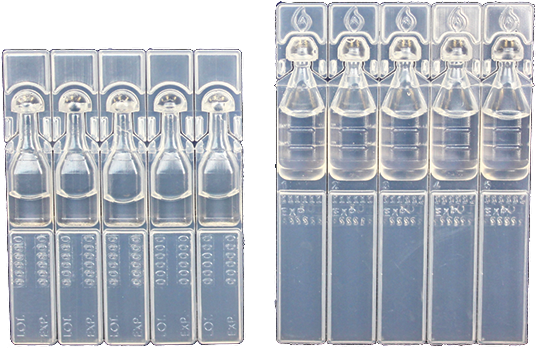
0.1mL - 1mL Vials
Up to 18,500/Hr
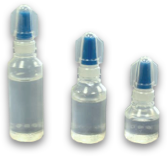
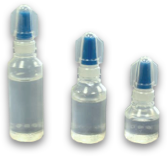
Eye Drops
50mL - 100mL Bottles
Up to 5,000/Hr IV Fluids
(Parenterals)

500mL Bottles
Up to 3,000/Hr IV Fluids
(Parenterals)

500mL Bottles
Up to 1,200/Hr
Humidifier


2000mL Bottles
Up to 800/Hr
Inhalation



2mL - 5mL Vials
Up to 15,000/Hr
Respiratory/Inhalation

0.1mL - 1mL Vials
Up to 18,500/Hr
Eye Drops

50mL - 100mL Bottles
Up to 5,000/Hr IV Fluids
(Parenterals)

500mL Bottles
Up to 3,000/Hr IV Fluids
(Parenterals)

500mL Bottles
Up to 1,200/Hr
Humidifier

2000mL Bottles
Up to 800/Hr
Inhalation
ALPS 600LN Series

ALPS 300LN Series